Ichthyophthirius multifiliis is a relatively large, single-celled ciliated protozoan that causes “Ich” or “white spot disease.” This disease is a major problem to freshwater aquarists and commercial fish producers worldwide. All species of freshwater fish are considered susceptible, and the parasite has been found in all areas of the world in both cultured and wild fish. Although large, these parasites do require a microscope to confirm them as a cause of the characteristic white spots that are often seen on the skin and fins of infected fish (Figure 1). The disease is highly contagious and spreads rapidly from one fish to another without the need for additional hosts (direct life cycle). Although outbreaks may occur at any time, they often appear when water temperatures are changing more rapidly. In the spring, when water temperatures are increasing, the Ich life cycle proceeds more quickly in warmer temperatures; conversely, in the fall and winter, decreases in fish immune function will also favor infection. The disease is particularly severe when fish are crowded. While many protozoans reproduce by simple division (one parasite “splits” into two), a single Ich organism can multiply into hundreds of new parasites in one generation, making early detection and treatment of this parasite crucial. The organism is unusual in that it is an obligate parasite, which means that it cannot survive unless live fish are present. Ich is capable of causing massive mortality within a short period of time. An outbreak of Ichis a true emergency situation and requires immediate treatment; if left untreated, this disease may result in 100% mortality.

Credit: R Yanong, UF/IFAS
Life Cycle
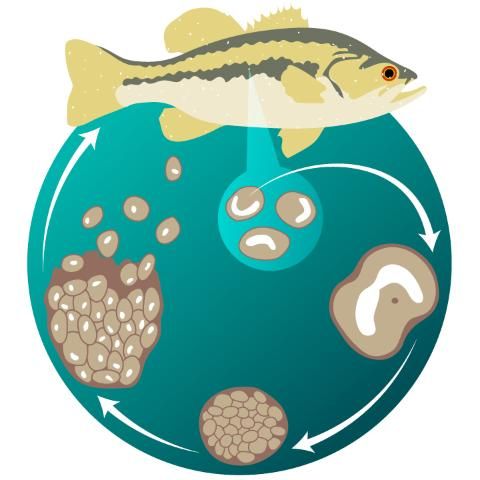
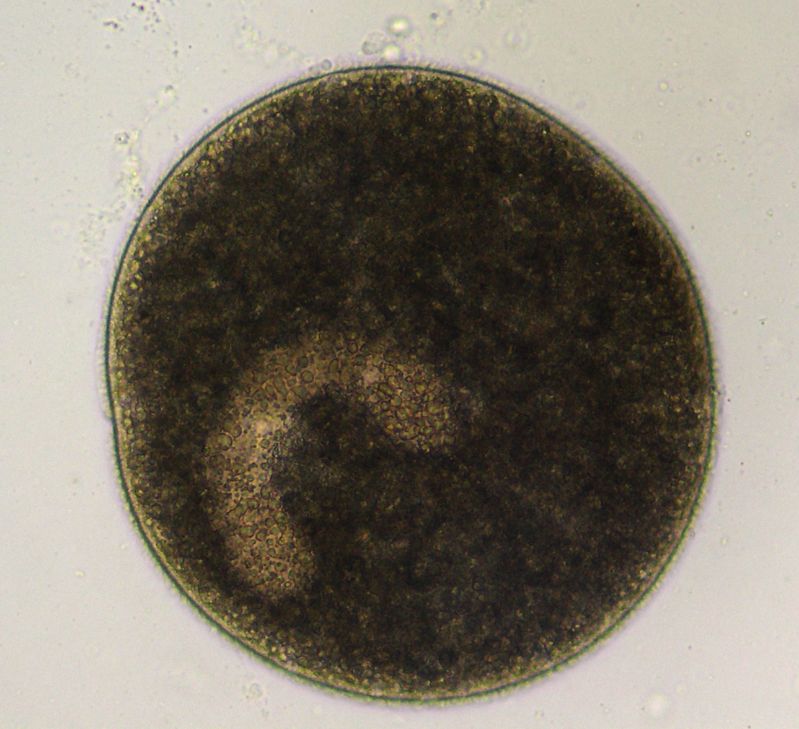
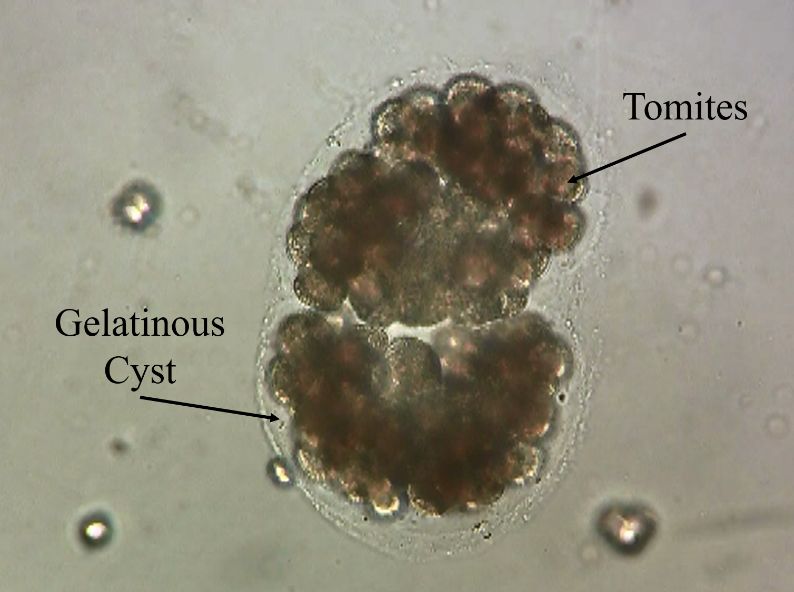
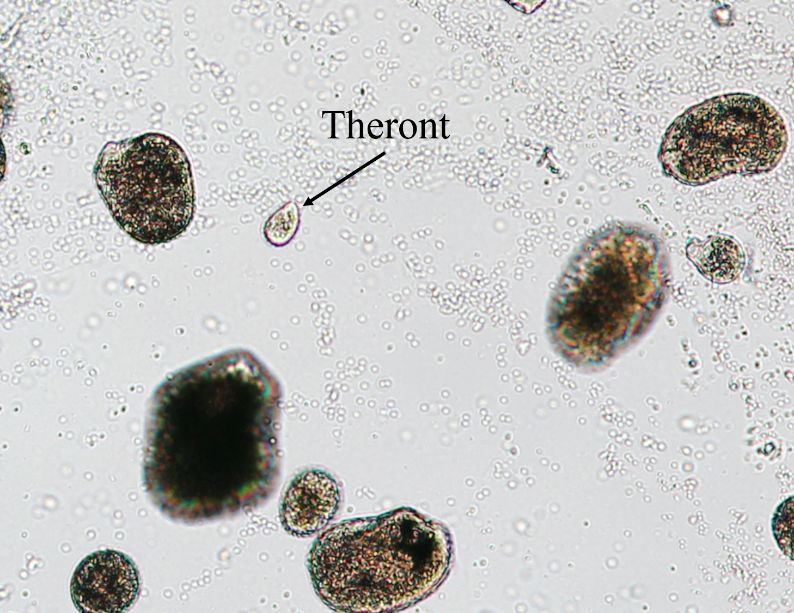
Although Ichthyophthirius multifiliis has a direct life cycle (i.e., it reproduces without a need for another animal species host) (Figure 2), the life cycle is complex and has three distinct life stages: 1) the on-fish, feeding trophont (Figure 3); 2) the environmental, reproducing tomont (Figure 4); and 3) the infective, fish-seeking theront (see Figure 5). The trophont invades and encysts between the thin outer layers (epithelium) of the fish host’s skin and gills to feed on those tissues (Figure 6). Because it is covered by the fish’s epithelial tissue and mucus, the adult parasite, or trophont stage, is protected from chemical treatment. Once the trophont is mature, it stops feeding, leaves the fish, and becomes a tomont. The tomont quickly secretes a gelatinous-walled outer cyst (Figure 4) that allows it to stick to surfaces in the environment. Some references consider the tomont to be the stage that leaves the fish, becoming a tomocyst after it settles and forms a cyst, but for purposes of EDIS we will use the more traditional life cycle as described in Figure 2. The tomont begins to divide quickly up to 10 times, forming as many as 1024 new “daughter” parasites (tomites) within a single cyst (Figure 4). This can occur in 18 – 24 hours at warmer water temperatures (23°C/74°F). The gelatinous wall of the tomont cyst protects it and the daughter tomites from chemical treatment. The tomites begin to develop and become theronts within the tomont cyst. Following a period of days (warm water temperatures) or weeks (cool water temperatures), the theronts bore out of the tomont cyst and become free-swimming, infective parasites in search of a fish host. These infective theronts must find a live fish to complete the parasite’s life cycle. This free-swimming phase is unprotected and, therefore, highly susceptible to chemicals. Treatment protocols should be designed to target this theront stage.
Credit: UF/IFAS
Credit: D. Pouder, UF/IFAS
Credit: D. Pouder, UF/IFAS
Credit: D. Pouder, UF/IFAS
Credit: D. Pouder, UF/IFAS
Disease Signs
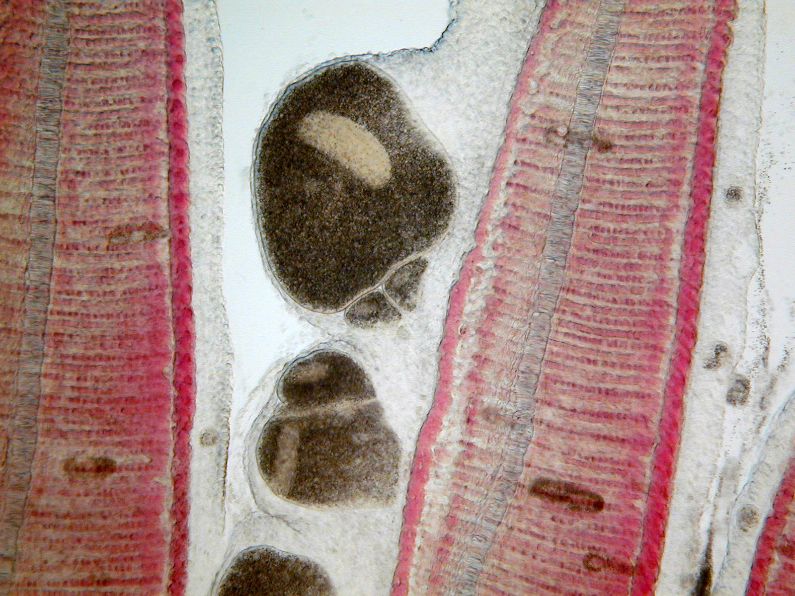
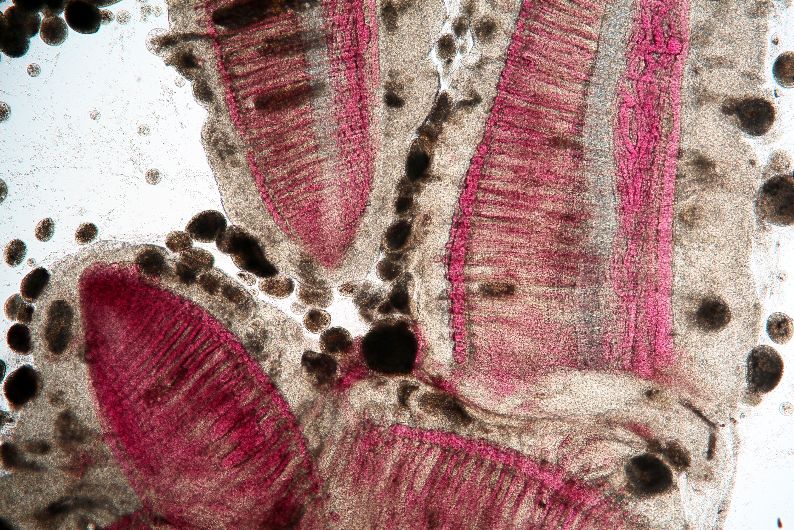
The classic sign of an Ich infection is the presence of small white spots on the skin or fins (Figure 1). These spots are caused as the adult parasite (trophont, Figure 3) penetrates and creates a space in the outer layers of the fish’s body surfaces (epithelium) to feed on the fish and move around. These lesions look like small white dots, blisters, or salt grains on the skin or fins of the fish. The white spots may not be as obvious on fish that are white or pale in color, or if the infection is limited to the gills. By the time the white spots are visible to the naked eye, the infected fish is very sick. Prior to the appearance of white spots, fish may have shown signs of irritation, flashing, increased mucus, weakness, loss of appetite, and decreased activity. A well-trained aquaculturist or aquarist will detect these changes before the fish’s condition worsens and mortalities occur. If the parasite is only present in the gills (Figures 6 and 7), white spots may not be seen at all but fish will die in large numbers. In these fish, gills will often be pale and very swollen. White spots should never be used as the only means of diagnosis because other diseases may have a similar appearance. Gill and skin biopsies should be collected and examined with a light microscope when the first signs of illness are observed.
Credit: D. Pouder, UF/IFAS
Diagnosis of Ich
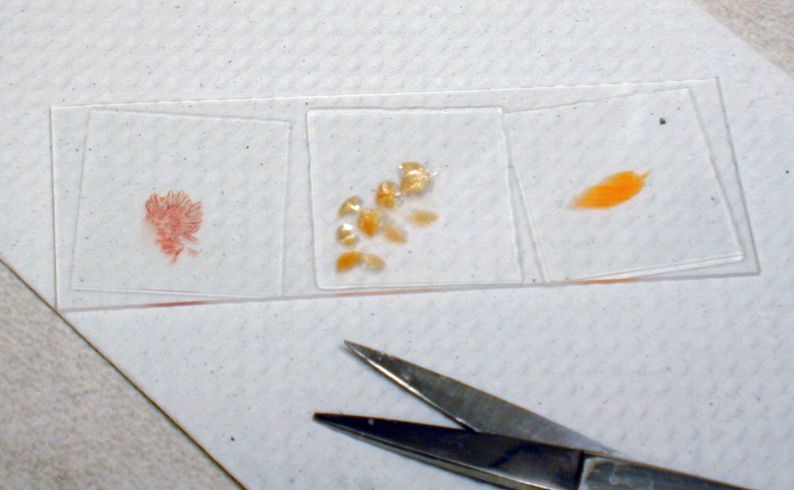
While Ich can be suspected by the typical appearance of white spots on some fish, a diagnosis requires confirmation by identification of the parasite in infected tissue using a compound microscope. To do this testing, a glass coverslip can be lightly scraped down the side of a fish (in the direction of head to tail) to remove some skin cells (preferably with some of the white spots) and mucus. A small area of gill and fin may also be carefully clipped using small, sharp scissors. Keep all tissue pieces small to minimize any harm to the fish. Small tissue pieces will also be easier to evaluate because it is very hard to view the parasites when examining thick tissue sections. Mount the skin, fin, and gill samples in separate drops of tank or other fresh water on a microscope slide and gently cover with a glass coverslip (Figure 8). (Do not use chlorinated tap water, reverse osmosis water, or distilled water. Bottled spring water can be an acceptable clean, fresh water source.)
Credit: D. Pouder, UF/IFAS
The mature trophont is large, oval to round, dark in color (due to the thick cilia covering the entire cell) and measures 0.5 to 1.0 mm in size. This stage also has a horseshoe- or C-shaped macronucleus that may be visible under 40x magnification (Figure 7). The parasite moves slowly in a rolling, sometimes amoeboid motion and, with practice, is easily recognized. The immature, free-swimming theronts (Figure 5) are smaller, pear- or spindle-shaped, translucent, and move quickly, continuously spinning on its longest axis as it swims. Theronts can resemble other parasites (especially Tetrahymena), so if only this juvenile stage is seen, prepare a second slide and examine it closely for the trophont stage to confirm the diagnosis. One Ich organism will produce up to 1024 individuals in one generation. Therefore, if even a single Ich parasite is seen, fish should be medicated immediately because the fish may not survive as the infection advances, even with treatment.
Prevention of Ich
Prevention is always preferable to treating Ich (or any disease) after an outbreak is in progress. Preventing introduction of this parasite is one of the most important reasons all incoming fish should be quarantined. Transport and handling can cause newly arrived fish who may be asymptomatic carriers (those with no obvious clinical signs) to break with active disease, serving as a source of infection for other fish they may come in contact with. At the warm water temperatures required for many aquarium fish, active disease will often become evident 1–3 weeks after shipping. For this reason, a minimum 30-day quarantine period is recommended for new fish. The importance of this quarantine period for aquaculture or public aquarium facilities cannot be over-emphasized. Additionally, because the environmental tomont cyst is sticky (Figure 4), it can easily spread between systems. For this reason, nets, siphon hoses, and other equipment that have not been disinfected should not be shared between tanks, especially in a quarantine area. Similarly, freshwater plants or other structures that may have been exposed to infected fish may carry tomont stages. Ich may also be spread by aerosolization of water mist or spray so nearby systems should be watched carefully. For more information on general biosecurity practices and disinfectants, please refer to USDA SRAC Publication 4707, Biosecurity in Aquaculture, Part 1: An Overview.
General Treatment Guidelines
Once an outbreak of Ich is detected, it is important that a treatment protocol be started immediately. Control of this parasite can be difficult because of its complex life cycle, multiple protected stages, and large number of off-spring from a single individual. The role of water temperature in determining the timing of treatment application is also critical and is discussed in more detail below. Of the life stages shown (Figure 2), only the free-swimming theronts are susceptible to chemical treatment. This means that the application of a single dose of a treatment will only kill theronts that have emerged from the tomont cyst and have not yet burrowed into the skin or gills of a host fish. This single treatment dose will not affect organisms that emerge after the chemical has broken down or been flushed from the system. Appropriately timed, repeated treatments, however, will continually kill the juvenile, infective theronts, preventing continuation of the infection. The disease outbreak will be controlled as more adult trophonts drop off the sick fish, encyst, and produce theronts that cannot survive the chemical treatment in the water. In a tank or vat, this process can be greatly enhanced if organic debris is removed following treatment. Because the sticky cyst of the tomonts may attach to organic material, cleaning this debris will help remove many cysts from the environment, further decreasing the number of emergent theronts. When removing debris, it is important that the debris or water not be discarded on the floor or anywhere that it could spread the parasites to a different tank or system. Any dead fish should be removed as soon as they are seen because mature trophonts will quickly abandon a fish once it has died and begin reproducing in the environment.
Water temperature has a tremendous influence on how fast the life cycle of Ich (Figure 2) is completed. At warm temperatures (75°F–79°F), the life cycle is completed in about 3 to 6 days. At these temperatures, chemical treatments should be applied daily, and a minimum of 3 to 5 treatments are required. At cooler temperatures the life cycle is prolonged, and treatments should be spaced further apart. For example, at a water temperature of 60°F, treatments should be spaced 3 to 5 days apart with a minimum of five treatments. Treatments should never be discontinued until all mortality from Ich has stopped, and re-testing of fish may be necessary.
Fish should be closely watched during recovery because the weakened fish may be susceptible to a secondary bacterial infection. Survivors of an Ich outbreak may also serve as reservoirs of infection. The immune system has been able to fight and control the parasite numbers enough that the fish will not show any clinical signs even if they have a lower level infection; however, they may be capable of spreading Ich to other fish that have not been previously exposed to the disease.
Chemical Treatment Options
The choice of chemical used to treat Ich will be based upon water quality conditions, species of fish to be treated, and the type of system in which the fish are housed. In general, copper sulfate and formalin are both effective against Ich when applied at the correct dose in a repetitive manner as described above. Increased salinity can also be effective.
Copper Sulfate
Most aquacultured channel catfish in the southeast United States are reared in ponds. For these fish, the treatment of choice for Ich is often copper sulfate. The chemical is effective and relatively inexpensive, an important consideration when large volumes of water are treated; however, it can also be used in smaller aquarium systems. It is approved by the Environmental Protection Agency (EPA) as an algaecide in aquaculture systems, and the Food and Drug Administration (FDA) currently classifies it as “regulatory action deferred” pending further study. The disadvantage of copper sulfate is that it is extremely toxic, particularly in water of low alkalinity. NEVER use copper sulfate without first testing the total alkalinity of the water, carefully measuring the dimensions of the system to be treated and weighing the amount of chemical to be applied.
The concentration of copper sulfate to apply in freshwater is calculated by determining the total alkalinity of the water and dividing that number by 100. For example, if the total alkalinity of the pond water is 100 mg/L, then 100/100 = 1 mg/L copper sulfate. Do not use copper sulfate if the total alkalinity is less than 50 mg/L. If you have never used copper sulfate, contact a UF/IFAS Extension aquaculture specialist for assistance. When treating smaller systems with copper sulfate, it is often advisable to create a stock solution of the chemical to facilitate delivery of very small amounts. Given the toxicity, over-dosing should be expected to kill fish. Because copper sulfate is an algaecide, its use may lead to severe oxygen depletions in systems with phytoplankton blooms or other plant populations; therefore, emergency aeration should always be available. Use of copper sulfate during hot or cloudy weather or when algae (phytoplankton) blooms are dense is strongly discouraged. Remember, if you do not know the alkalinity of your water and cannot measure it, DO NOT USE COPPER SULFATE. For more detailed information on the use of this chemical, please refer to University of Florida EDIS fact sheet FA-13, Use of Copper in Freshwater Aquaculture and Farm Ponds.
Formalin
If fish are maintained in a tank system, formalin is often used to treat Ich. Formalin is not the ideal treatment for ponds, but it works well in tanks. Vigorous aeration should be supplied during chemical treatments, especially if gills have been damaged. Parasite-S (Syndel) and Formacide-B (B.L. Mitchell, Inc.) are formalin products approved by the Food and Drug Administration (FDA). These two products are approved to treat external parasites on all species of fish at all life stages. Formalin is usually applied at a concentration of 25 mg/L, which is equivalent to 1 ml of formalin per 10 gallons of system water. For formalin-sensitive species, a “half-dose” of 12.5 mg/L (0.5 ml formalin per 10 gallons of water) may be used.
If the system is flow-through (new water continuously being added to the system), the incoming water should be turned off for at least 12–18 hours (and up to 24 hours). (This method would not be appropriate for a heavily stocked, high-flow raceway system such as those used for salmonid production. In addition to chemical treatment, cleaning the tank will also decrease the number of parasites. Sick fish may be unable to tolerate a full treatment. If they appear stressed or try to jump out of the tank, flush the chemical from the system immediately with clean, well-oxygenated water. For more detailed information on the use of this chemical, please refer to UF/IFAS EDIS fact sheet VM-77, Use of Formalin to Control Fish Parasites.
Adjusting Salinity
A slight increase in salinity can help decrease osmoregulatory stress caused by the damage this parasite causes to the external tissues of the fish. At warmer water temperatures (75–79°F), use of 4–5 g/L (= 4–5 ppt) salt (sodium chloride) in a prolonged bath for 7 to 10 days is another effective treatment in smaller systems, provided the fish species can handle the increased salt concentration. Because theronts are intolerant to increased salinity levels of 3–5 ppt, salt is often added to aquaria or tanks that are being treated with formalin to enhance the response to treatment. Most freshwater fish can tolerate 5 ppt salinity for several weeks and many can live in 3 ppt permanently; however, it is important to know the specific tolerances for each species to be treated. Plants in an aquarium or small ornamental pond may not tolerate increased salinity.
Avoid Potassium Permanganate
Although potassium permanganate is a good choice for many external fish parasites, the need for repeated treatments in a short period of time make it a more dangerous choice for control of Ich. Potassium permanganate is a strong oxidizer, and its use more than once a week is discouraged to prevent damage to the skin, gills, and eyes of the fish. For these reasons it is not recommended as a treatment for Ich.
Special Considerations for Treatment of Pet Fish
Pet fish can be treated with any of the chemicals discussed above to alleviate Ich infections. Several commercial preparations are available from pet stores that contain one or more of these agents. In addition to chemical treatments, cleaning the tank every other day will help remove cysts attached to debris before the theronts emerge thereby helping prevent reinfection of the fish and completion of the life cycle.
Summary
Ichthyophthirius multifiliis (Ich) is a common, ciliated protozoan parasite that can cause catastrophic losses in aquaculture facilities and display aquariums. The most characteristic features of the mature trophont stage of the parasite are a continual rolling, amoeboid motion and a horseshoe-shaped macronucleus, both of which are easily recognized during a microscopic examination of infected tissue. However, because mature trophonts may not be the only stage observed, all individuals may not have the obvious macronucleus.
Ich is easily introduced into a fish pond, tank, or home aquarium by new fish, substrate, plants, decorations, structures, or equipment that has been moved from one fish unit to another. Quarantine and, if necessary, treatment of new fish is an effective way of preventing spread of this disease. Similarly, holding substrates and live plants with no fish for the duration of the Ich life cycle at a given temperature will also help prevent infection. Decorations or structures coming from another aquarium with fish should be cleaned and disinfected appropriately prior to use.
Once the organism gets into a system, it can cause massive mortality of fish within a short time and, in severe cases, control may be impossible and 100% mortality of fish can be expected. In contrast to most parasitic diseases where the decision to treat (or not to treat) is based on the degree of infestation and other factors, fish infected with even a single Ich parasite should always be treated immediately because of its explosive reproductive rate. A single treatment is not sufficient for this disease because the life stage on the fish (trophont) and the stage encysted in the environmental (tomont) are resistant to chemicals. Only the infective theront stage is susceptible to treatment. Repeating the appropriate chemical treatment or prolonged use of salt as recommended above will disrupt the life cycle and control the outbreak. Daily cleaning of tanks is also beneficial because tomonts attached to organic matter can be physically removed from the environment. Fish that have survived an Ich infection are known to be potential reservoirs of the parasite and may cause other fish to become infected.
With quick response and careful treatment, Ich can be controlled, but the cost will be high for larger systems/facilities, both in terms of lost fish, labor, and the cost of chemicals. Careful attention to management including good biosecurity practices such as quarantine, avoiding tank to tank transmission, and multiple treatments when outbreaks occur, will minimize economic loss from this disease.
Recommended Reading
UF/IFAS Fact Sheet FA-13. Use of Copper in Freshwater Aquaculture and Farm Ponds. https://edis.ifas.ufl.edu/publication/fa008
UF/IFAS Fact Sheet VM-77. Use of Formalin to Control Fish Parasites. https://edis.ifas.ufl.edu/publication/VM061
USDA SRAC Publication No. 4707. Biosecurity in Aquaculture, Part 1: An Overview. http://fisheries.tamu.edu/files/2013/09/SRAC-Publication-No.-4707-Biosecurity-in-Aquaculture-Part-1-An-Overview.pdf
References
Dickerson, H. W. 2006. "Ichthyophthirius multifiliis and Cryptocaryon irritans (Phylum Ciliophora)". Pages 116–153 In P. T. K. Wood, editor. Fish diseases and disorders – volume 1: protozoan and metazoan infections, 2nd edition. CAB International, Cambridge, Massachusetts. https://doi.org/10.1079/9780851990156.0116
Dickerson, H. W. 2012. "Ichthyophthirius multifiliis". Pages 55–72 In P. T. K. Wood and K. Buchmann, editors. Fish parasites: pathobiology and protection. CAB International, Cambridge, Massachusetts. https://doi.org/10.1079/9781845938062.0055
Durburrow, R.M., Mitchell, A.J., Crosby, M.D. 1998. Ich (White Spot Disease). Southern regional Aquaculture Center, Publication No. 476. 6pp.
Noga, E. J. 2010. Fish disease: diagnosis and treatment, 2nd edition. Wiley-Blackwell, Ames, Iowa. https://doi.org/10.1002/9781118786758